As a typical transition metal sulfide, molybdenum disulfide (MoS2) has a good application prospect as a negative electrode material for lithium ion batteries due to its unique S-Mo-S two-dimensional layered structure and high active sulfur content. When assembling a half battery with lithium metal, MoS2 stores lithium through an "intercalation - conversion" method and produces lithium sulfide and elemental molybdenum at the end of the first turn discharge, with a theoretical specific capacity (~670 mA h g-1) twice that of the currently widely used graphite negative electrode. However, the low conductivity and electrochemical instability of MoS2 are not conducive to its cycle and rate performance. In addition, after the first cycle, the MoS2 layered structure rearrangement is easy to form larger bulk phase particles, so that it cannot react adequately in the subsequent lithium/delithium process, resulting in rapid attenuation of material capacity, and affecting its application in actual batteries.
In order to solve the above problems, it has been proposed to combine MoS2 with a conductive carbon substrate to improve the electrochemical performance of the material. By evenly dispersing the nano-state MoS2 on the surface of the active site of the carbon substrate, it is expected that the lithium ion and electronic conductance of the composite can be significantly improved, and the electrochemistry of MoS2 can be stabilized through the pinning effect or strong interaction between the sulfide and the carbon, and the agglomeration of the sulfide can be prevented during the cycle. In terms of the selection of carbon substrates, graphene is an ideal material for dispersive nano-MOS2 due to its significant advantages such as large specific surface area, high conductivity and good flexibility. In the nanoscale composite structure optimization of MOS2-graphene composite materials, it is necessary to take into account the electrochemical stability and dynamic advantages of the material, at the same time, in order to improve the energy density of the composite material, it is also necessary to consider the compaction density of the material.
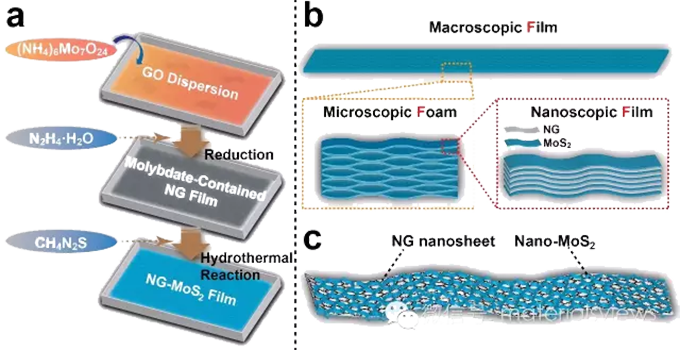
Recently, Professor Huai Ping's research group at Hefei University of Technology and Professor Yu Shuhong's research group at the University of Science and Technology of China have realized the self-assembly design and scale-up preparation of a self-supporting molybdenum disulfide graphene composite film. The film can be prepared by hydrazine hydrate steam reduction combined with hydrothermal reaction. The basic structural units of the film include nitrogen-doped graphene (NG) and honeycomb nano MoS2 (NG-MOS2), and the macro-micro-nano hierarchical structure of "film - foam - film" is displayed from top to bottom. When used as anode materials for lithium-ion batteries, this new structural design can ensure that the composite material has a high compaction density, and can ensure the rapid transport of lithium ions and electrons inside the material, while also accommodating the volume change of the sulfide material during the process of lithium removal. Due to its significant advantages in structure, NG-MoS2 composite material shows excellent electrochemical performance when used in the negative electrode of lithium-ion battery. Its reversible lithium storage capacity reaches 1200 mA h g-1 at 0.1A g-1 current density, and the specific capacity remains 700 mA h g-1 when the current density rises to 5.0A g-1. After 400 cycles at 1.0A g-1 current density, the specific capacity remains 980mA h g-1. Given the significant advantages of MoS2 in terms of capacity compared to traditional lithium anode materials such as graphite, this NG-MoS2 composite anode material is expected to show good application prospects in energy storage systems represented by the next generation of lithium-ion batteries and contribute to the development of sustainable energy technologies for the future.
Relevant results have been published recently in Angew. Chem. Int. Ed. (DOI: 10.1002 / anie. 201606870) and was elected to the VIP.
From: MaterialsViews
